
Registered customer
wrong mail adress or password

Forgot Password?

Open Account
Fejl i captcha. Prøv igen

Registered customer
wrong mail adress or password

Forgot Password?

Open Account
Fejl i captcha. Prøv igen
Limmets hårdhet mäts i Shore A och Shore D, men vad betyder siffrorna.
Se en grafisk förklaring av de olika hårdheterna
Godkända lim för dricksvatten och livsmedel, läkemedelsgodkända och mycket annat. Se godkännanden här
Limmets viskositet mäts i mPa·s, men vid vilken viskositet är limmet tunt eller trögflytande?
Se våran QuickGuide om viskositet
Limmets hårdhet mäts i Shore A och Shore D, men vad betyder siffrorna.
Se en grafisk förklaring av de olika hårdheterna
Godkända lim för dricksvatten och livsmedel, läkemedelsgodkända och mycket annat. Se godkännanden här
Limmets viskositet mäts i mPa·s, men vid vilken viskositet är limmet tunt eller trögflytande?
Se våran QuickGuide om viskositet
Limmets hårdhet mäts i Shore A och Shore D, men vad betyder siffrorna.
Se en grafisk förklaring av de olika hårdheterna
Godkända lim för dricksvatten och livsmedel, läkemedelsgodkända och mycket annat. Se godkännanden här
Limmets viskositet mäts i mPa·s, men vid vilken viskositet är limmet tunt eller trögflytande?
Se våran QuickGuide om viskositet
Limmets hårdhet mäts i Shore A och Shore D, men vad betyder siffrorna.
Se en grafisk förklaring av de olika hårdheterna
Godkända lim för dricksvatten och livsmedel, läkemedelsgodkända och mycket annat. Se godkännanden här
Limmets viskositet mäts i mPa·s, men vid vilken viskositet är limmet tunt eller trögflytande?
Se våran QuickGuide om viskositet
Limmets hårdhet mäts i Shore A och Shore D, men vad betyder siffrorna.
Se en grafisk förklaring av de olika hårdheterna
Godkända lim för dricksvatten och livsmedel, läkemedelsgodkända och mycket annat. Se godkännanden här
Limmets viskositet mäts i mPa·s, men vid vilken viskositet är limmet tunt eller trögflytande?
Se våran QuickGuide om viskositet
Limmets hårdhet mäts i Shore A och Shore D, men vad betyder siffrorna.
Se en grafisk förklaring av de olika hårdheterna
Godkända lim för dricksvatten och livsmedel, läkemedelsgodkända och mycket annat. Se godkännanden här
Limmets viskositet mäts i mPa·s, men vid vilken viskositet är limmet tunt eller trögflytande?
Se våran QuickGuide om viskositet
Limmets hårdhet mäts i Shore A och Shore D, men vad betyder siffrorna.
Se en grafisk förklaring av de olika hårdheterna
Godkända lim för dricksvatten och livsmedel, läkemedelsgodkända och mycket annat. Se godkännanden här
Limmets viskositet mäts i mPa·s, men vid vilken viskositet är limmet tunt eller trögflytande?
Se våran QuickGuide om viskositet
Limmets hårdhet mäts i Shore A och Shore D, men vad betyder siffrorna.
Se en grafisk förklaring av de olika hårdheterna
Godkända lim för dricksvatten och livsmedel, läkemedelsgodkända och mycket annat. Se godkännanden här
Limmets viskositet mäts i mPa·s, men vid vilken viskositet är limmet tunt eller trögflytande?
Se våran QuickGuide om viskositet
Liquids are made up of many molecules that attract each other. This cohesive force at the surface of a liquid creates what is called surface tension. The magnitude of this property can be either high or low, depending on the strength of the molecular attraction. For example, water has a relatively high surface tension, which is why a small dome can form on top of water in a filled glass.
When it comes to bonding, it is more difficult to glue surfaces with low surface tension. For instance, as shown in the graph below, Teflon (which is used on cookware) is very difficult to bond. Taking silicone bonding as an example, silicone has a very low surface tension (around 24 mN/m). When a material has a lower surface tension than the adhesive being used, bonding will generally not succeed. Therefore, silicone surfaces must be pre-treated, for example by plasma treatment, corona treatment, or by applying a liquid primer to the surface before the adhesive is applied.
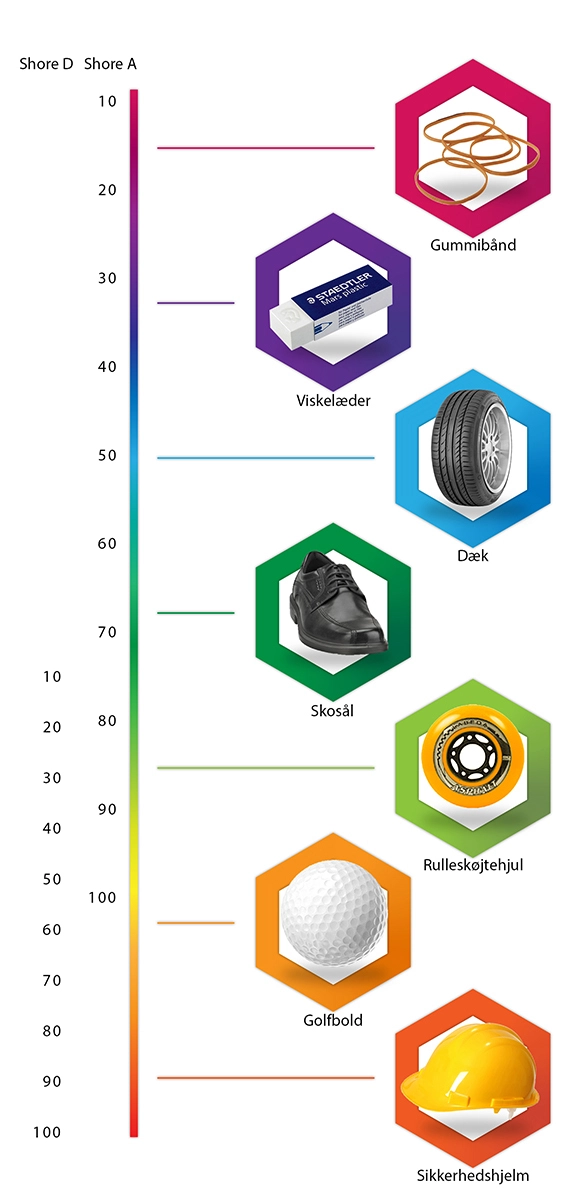
Limenes hårdhed efter udhærdning måles oftest enten i enheden Shore A eller Shore D, som begge har en skala fra 1-100.
Nedenfor kan se du en række eksempler på de forskellige måleenheders hårdhed.
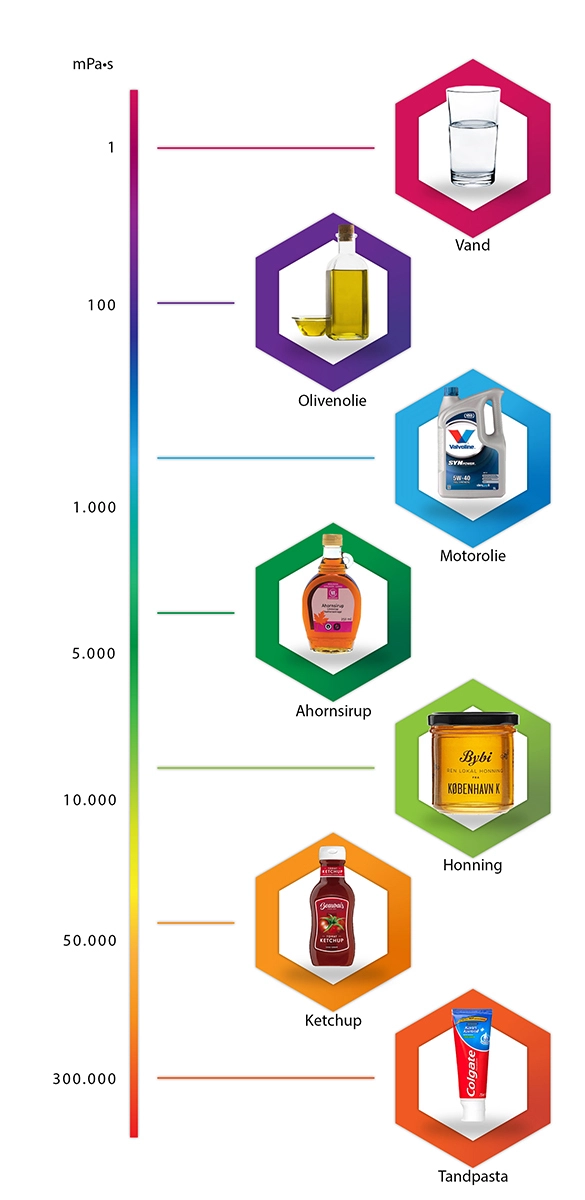
I forbindelse med beskrivelse af limens egenskaber forekommer begrebet viskositet, men hvad betyder det ? Viskositet er et begreb der anvendes til at beskrive limens træghed, altså hvor tyndflydende eller tykflydende en lim er. Viskositeten måles oftes ved omrørirng af en væske. Den kraft der skal bruges til omrøringen udtrykkes som Pascal. Det er naturligvis vigtigt at have for øje, at forskellige medier ændred viskositet alt efter om det er varmt eller koldt. En væske bliver mere for eksempel tyndflydende når den er varm.
Vores lime er alle mål i mPa·s (millipascal sekund)
Nedenfor kan du se en række eksempler på kendte medier og deres viskositet. Eksempelt starter ved vand, som har en viskositet på 1 mPa·s.
...to remember your settings, perform traffic measurements, and show you targeted content and ads. By clicking “Accept all cookies” you consent to all types of cookies, but you can change your settings or withdraw your consent at any time by clicking “Cookie settings.” You can read more about our use of cookies in our “Cookie Policy.”
Necessary
Necessary cookies help make a website usable by enabling basic functions such as page navigation and access to secure areas of the website. The website cannot function optimally without these cookies.
Functional
We collect information about your preferred settings and choices on the website. We do this to show you the version of the website that matches your preferences. The information is used to determine which region and language you prefer, and to display videos and other visual elements on the website, e.g., job search.
Marketing
We collect information about your interests, including which pages and ads you click on and which products or services you show interest in or purchase, on this and other websites. We do this to show you ads that are relevant to you and your interests. To show you targeted ads on this and other websites, we work with other companies with whom we share information. You can read more about this below.
You are warmly welcome to read our articles and product descriptions. Please note that we currently only serve business customers.